Explain Three Differences Between Exothermic and Endothermic Reactions
Gibbs free energy is the energy used by a substance in a chemical transformation or reaction. The balance between the two dictates whether a chemical reaction is overall exothermic bond forming step is bigger or endothermic bond breaking step is bigger.
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples
A 1 x 10³moldm³ and b 6 x 10⁹moldm³ What is the pH of each solution5.
. Explain and give an example each of a Hygroscopy b Efflorescence c Deliquescence4. A Give three crystalline and four non-crystalline allotropes of carbon b Give three differences between the two main crystalline. Learn the formula used to calculate Gibbs free energy and how a.
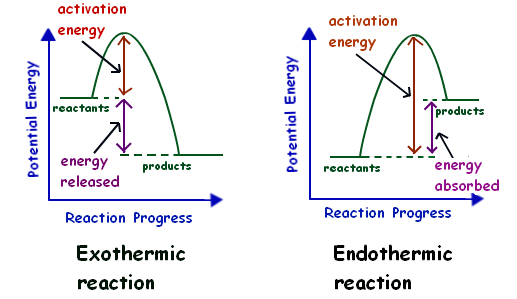
If a reaction is endothermic then enthalpy can be thought of as entering the reaction on the reactant side and if a reaction is exothermic heat can be thought of as exiting the reaction on the product side. The concentration of hydrogen ion H in two solutions are. Open Menu Close Menu.
Virtual lab 5 types of chemical reactions answer key.

Difference Between Endothermic And Exothermic Reaction Brainly In

Differentiate Between Exothermic And Endothermic Reaction

7 Difference Between Exothermic And Endothermic Reaction With Examples Viva Differences
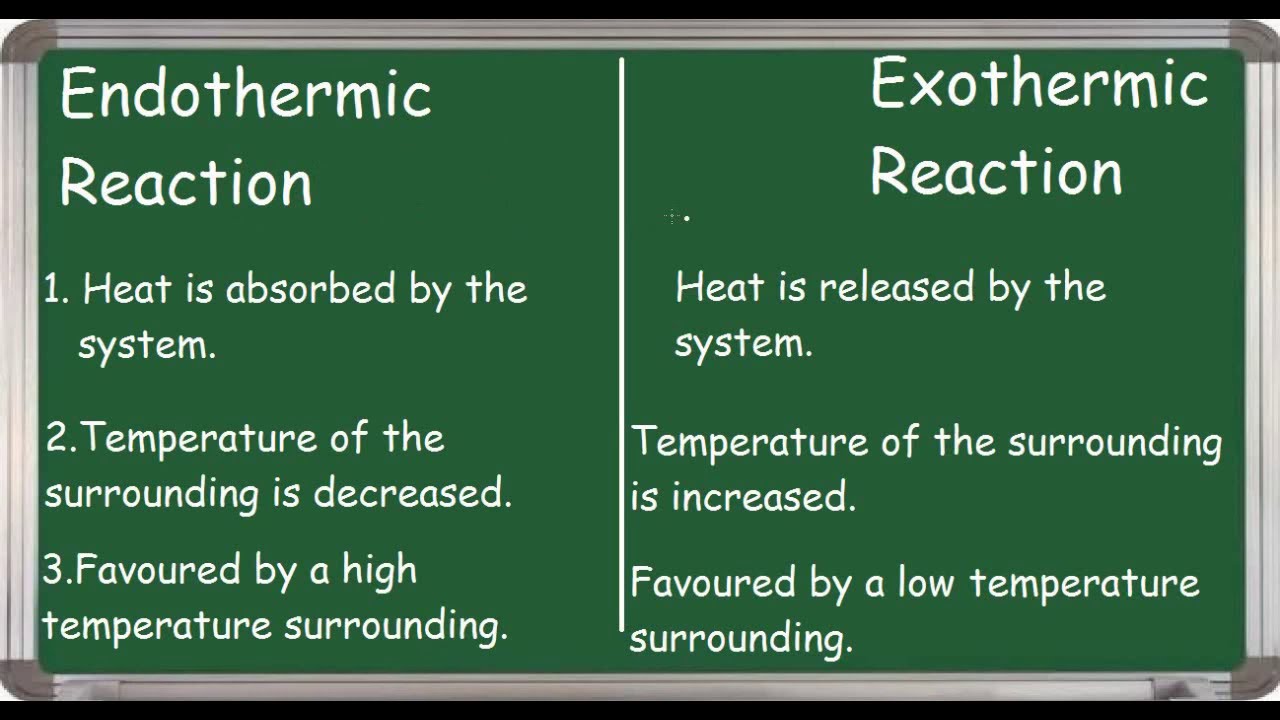
Difference Between Endothermic And Exothermic Reactions Chemistry
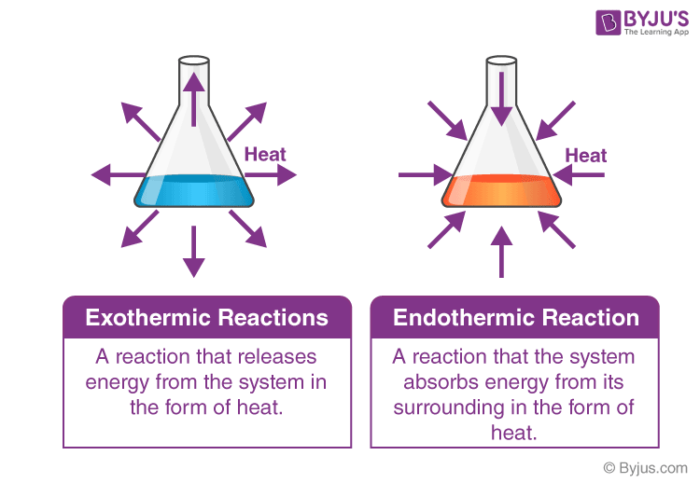
Difference Between Exothermic And Endothermic Reactions Diferr
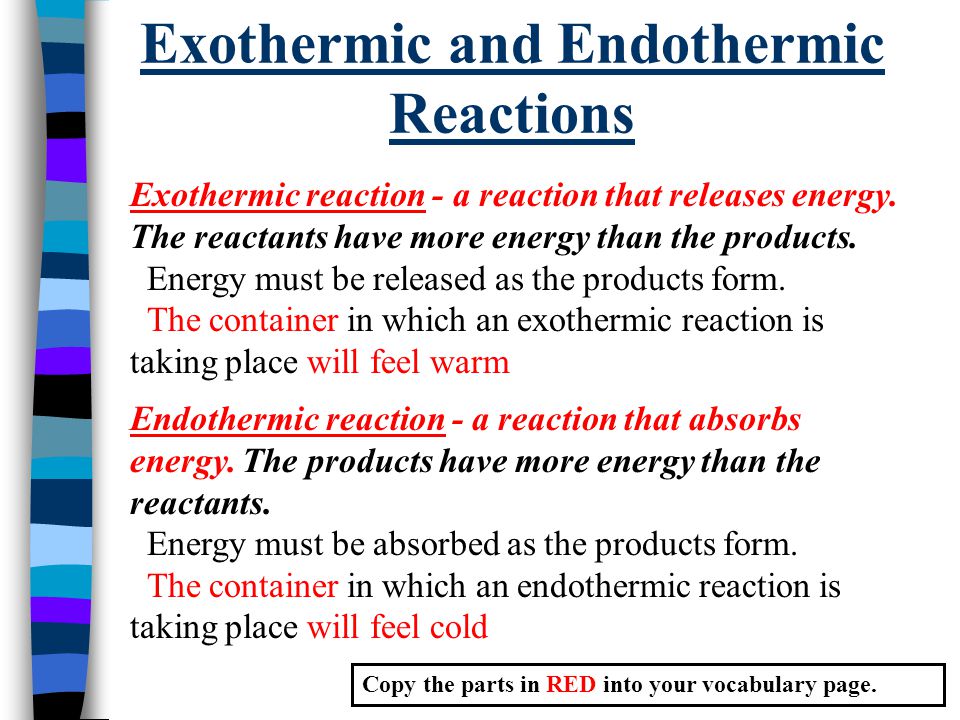
Key Question What Is The Difference Between Exothermic And Endothermic Reactions Warm Up Name 2 Ways You Could Speed Up A Chemical Reaction Ppt Video Online Download

Endothermic Vs Exothermic Reaction Differences Youtube

Differentiate Between The Following Exothermic Reaction And Endothermic Reaction

Difference Between Endothermic And Exothermic Reactions Differbetween
No comments for "Explain Three Differences Between Exothermic and Endothermic Reactions"
Post a Comment